Antimony
Do you use makeup in your daily life? Whether you put it on when you go to a party, for a play you’re in, or to complete your Halloween costume, our metal of the month might be of interest for you. Antimony compounds have been used since ancient times: Egyptian women used stibnite, antimony sulphide known as kohl, as a cosmetic to blacken their eyes and eyebrows.
Historically, the ancients thought that this powder, derived from crushed antimony, helped cure eye infections. This practice is still seen in Yemen and in other countries.
Applications
Because of its excessive brittleness and the difficulty of shaping it, antimony (Sb) has no direct applications, but is extensively used as an alloying element to improve the hardness and strength of other metals. So antimony is present in lead-acid batteries to harden the lead of which the electrodes (grids) are made. Hard lead contains 15-25% Sb. In addition, type metal contains 60% Pb, 20-30% Sb and 10-20% tin, but this application has virtually disappeared as a result of the development of the offset printing process.
Antimony is used in the electronics industry to make certain types of semiconductor devices, such as infrared detectors and diodes.
It is also present in certain Pb–Sn–Sb anti-friction metals used to make bearings for large compressors and propeller shafts. The sulphide Sb2S3 is one of the components of brake linings. The intermetallic compounds AsSb, GaSb and InSb have found some applications in electronics. The main outlet -– accounting for around 50% of total antimony consumption - is in the form of the oxide Sb2O3, which is used to render fabrics, plastics, paints and other substances fireproof. Antimony compounds act as a co-synergist with halogenated flame retardants to enhance their effectiveness. Its properties make it possible for plastics to be used in applications where they would melt under normal circumstances, such as in computer casings and televisions.
Umicore Precious Metals Refining produces sodium antimonate, a compound used in certain special glasses, mostly solar PV glass. As a fining agent, antimony ions interact with oxygen, suppressing the tendency of the latter to form (large) bubbles.
Recycling
Generally speaking, the main end-of-life recycling option is from spent lead-acid batteries, where antimony is directly recovered as a lead-antimony alloy.
At Umicore Precious Metals Refining in Hoboken (Belgium), antimony is recovered from complex lead-bearing concentrates as well as various complex residues from the lead/copper/zinc industry. The antimony is extracted during the lead refining process in the form of sodium antimonate.
Properties
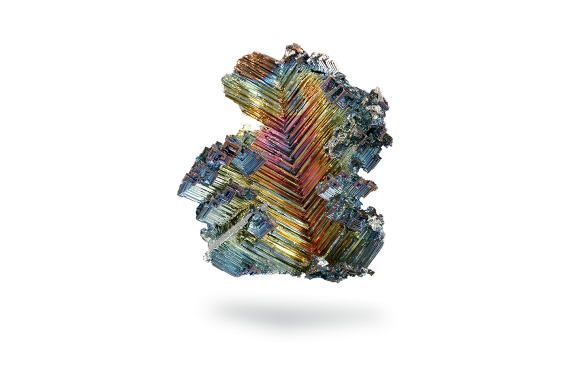
Antimony is a very hard, brittle and bluish-white metal with a highly distinct crystalline texture. Antimony is not an abundant element but is found in small quantities in over 100 mineral species. It is most often found as antimony(III) sulfide but also occurs as the native metal.
Like arsenic, there are various allotropes, one of which, yellow antimony, occurs only at temperatures below –80°C and is liable to explode.
Another allotrope, black antimony, crystallizes in the rhombohedral system and exhibits a metallic luster but is extremely brittle and much less volatile than arsenic.
History
Stibnite, the antimony sulphide Sb2S3, was known in ancient times. A cast antimony vase dating back to 4000 BC has been found in Chaldea, and antimony-coated copper articles were used in Egypt between 2500 and 2200 BC.
Antimony became widely used in the Middle Ages, mainly to harden lead for type.
It is not known exactly who isolated the metal for the first time, but antimony acquired considerable acclaim in 1707 when the French chemist Lémery published his Traité de l’Antimoine.
Its name is derived from two Greek words: anti - which means not - and monos - which means alone - but its symbol (Sb) comes from the Latin name 'stibium' meaning stain. The flower petal aspect that is observed on the natural sulphide varieties explains the etymology of the word antimony, derived from the Greek ανθος (flower).